Stem Cell Derived β-cell 3D Culture and Transplantation
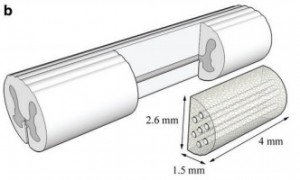
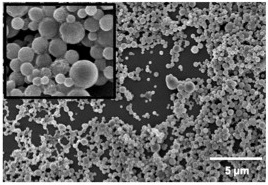
Type I diabetes (T1D) is caused by autoimmune destruction of pancreatic β-cells that results in the need for life-long insulin therapy. Although insulin therapy has improved the lives of those living with T1D, these individuals still experience hypoglycemic events, vascular complications, and elevated HbA1C. The recent clinical successes using islet transplantation have demonstrated the potential for cell replacement to improve glucose control in type 1 diabetics. The current clinical approach delivers islets through the portal vein, where they reside within the sinusoids of the liver. This method of allogeneic islet transplantation has several therapeutic limitations including a shortage of donor islets, long-term immunosuppression, and high risk of graft failure. These limitations have led to the investigation of new cell sources, and methods to support transplanted cells through tissue engineering, immunomodulation, and revascularization. Human pluripotent stem cells (hPSC), which can self-renew in culture and be differentiated into functional β-cells, could be the cell source needed to satisfy the demand for insulin producing cells. We are developing a transformative approach to the transplantation of hPSC-derived insulin-producing β cells using engineered microporous scaffolds. These scaffolds are used as a platform for pre-transplantation 3D culture, delivery into a clinically translatable transplant site, and post-transplantation cellular support. These microporous, tunable scaffolds have been designed to assemble hPSC-derived pancreatic progenitor cells into 3D islet-like structures, and promote their differentiation and function in vitro and in vivo. Our lab leverages expertise in systems biology and the use of non-destructive imaging techniques to track transcription factor networks, metabolic activity, and the production and secretion of insulin. Additionally, these scaffolds provide the means to locally control the transplant microenvironment through gene delivery and local biomolecular signaling to stimulate revascularization and locally modulate the activation of the immune response. This multi-disciplinary research exists at the intersection of tissue engineering, developmental and systems biology, and biomaterials and aims to develop a clinically translatable approach to cellular replacement therapy.
Researchers: Dan Clough, Feiran Li, Jessica King, Kelly Crumley, Liz Bealer
Cancer Metastasis and Tumor Cell Recruitment
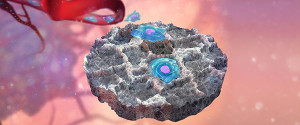
For many types of cancer, the formation of distant metastasis marks the disease stage in which curative treatment is no longer possible, and disease progression leads to mortality. At present, there are limited methods to detect metastases before they become radiologically evident or symptomatic. The spread of cancer from a primary tumor to distal sites is a highly intricate process requiring a myriad of cells within the microenvironment of the primary tumor and pre-metastatic niche, further complicated by the fact that early metastatic sites are difficult to detect and study. We are investigating metastatic progression using our novel biomaterial scaffold that recruits metastatic cancer cells and acts as a synthetic metastatic niche. This technology allows us to detect metastatic cells at an early stage, which could allow for life-preserving interventions, and to analyze the biology of metastatic cells as well as their stromal/immune microenvironment. By studying the dynamics of the metastatic niche during disease progression, we aim to monitor the efficacy of clinical treatments (including immunotherapy) and identify novel therapeutic targets for metastasis. We additionally combine systems biology techniques with our transcription factor activity reporters to elucidate intercellular circuits responsible for driving metastatic progression. The development of an implant to recruit metastatic cells and monitor disease progression could transform both our understanding of metastatic niche maturation and current clinical approaches to treat metastatic disease.
Researchers: Ravi Raghani, Jeffrey Ma, Sophia Orbach, Jing Wang, Aaron Morris, Ian Schrack, Guillermo Escalona
Regenerative Medicine: Spinal Cord Regeneration
Spinal cord injuries (SCI), resulting in loss of function such as paralysis below the level of injury, affect thousands of patients each year. The consequences tend to be life-long. A SCI can result in massive cell death and produce a cyst and glial scar that pose barriers to regeneration. Pioneering studies done in the 1980’s identified that central nervous system neurons have the capacity for regeneration; however, previous strategies for augmenting nerve regeneration have met limited success. Our lab has developed polymer scaffolds, termed bridges, that can be implanted into the spinal cord to create a more permissive environment for regeneration. The bridge is highly porous to allow for cell infiltration that stabilizes the surrounding tissues, and has channels that orient and direct axonal elongation. To further modulate neuroinflammation, regeneration, and glial scar formation, the delivery of gene therapy vectors via the bridge provides a versatile tool to target a range of processes. Recovery of function can be seen in both behavioral tests on awake animals and electrophysiological studies using optogenetic stimulation in anesthetized animals. Currently, we are moving towards implementing temporal control of gene therapy expression for translation and circuit analyses to examine the specificity of regenerated synapses.
Researchers: Sarah Hocevar, Brian Ross
Regenerative and Tolerogenic Immunomodulation
Modulating the immune response with vaccines has been one of the greatest success stories in the history of medicine. New tools to modulate the response of specific immune cell populations could facilitate novel therapies for autoimmune disease and regenerative medicine, and we are developing distinctive capabilities aimed at promoting i) a less inflammatory and more regenerative phenotype for regenerative medicine, or ii) antigen-specific tolerance, which has applications for autoimmune disorders, cell transplantation therapies, and protein therapeutics. In vivo reprogramming of immune cells: Strategies for controlling innate immunity (e.g., macrophage activation) are a crucial first step in promoting regeneration or inducing tolerance. Infiltrating immune cells, such as macrophages, are a promising target for inhibiting the degenerative inflammatory response after SCI because they can be shifted from an inflammatory to a regenerative phenotype to downregulate inflammatory cytokine production, and upregulate scavenger receptor expression for enhanced clearing of inhibitory debris. Gene delivery from the biomaterial scaffolds leads to transfection of macrophages, which are investigating as a mechanism to reprogram these cells into assets for regeneration or cell engraftment. Technologies for induction of antigen specific tolerance: Tolerance research emerged from our success and collaborations with islet transplantation for type 1 diabetes (T1D), and has extended to autoimmune disorders and allogeneic cell transplantation. We have collaborated on the production of antigen-coupled nanoparticles for the induction of tolerance to inhibit the specific undesired immune response while not altering the remaining elements of the immune response. Nanoparticles are modified with specific antigens, which target the spleen following intravenous delivery and are internalized by ‘tolerogenic’ APCs to present the antigens without T cell activation thereby inducing tolerance rather than an immune response. Most strategies targeting the immune system focus on inducing a specific immune response (vaccines), whereas this system aims to do the opposite by inducing tolerance to specific antigens. Interestingly, tolerance induction was enhanced by islet delivery on scaffolds relative to hepatic transplantation, suggesting the local environment impacts tolerance. PLG nanoparticles modified with the antigen have been developed that are able to prevent the development of autoimmune disease. Particles without peptide, or modified with the control peptide did not prevent disease. Ongoing research is investigating the particle design that can lead to tolerance, as well as employing the cell array to investigate the effect of particles on the differentiation state of macrophages.
Researchers: Eiji Saito, Aaron Morris
Systems Biology: Living cell arrays
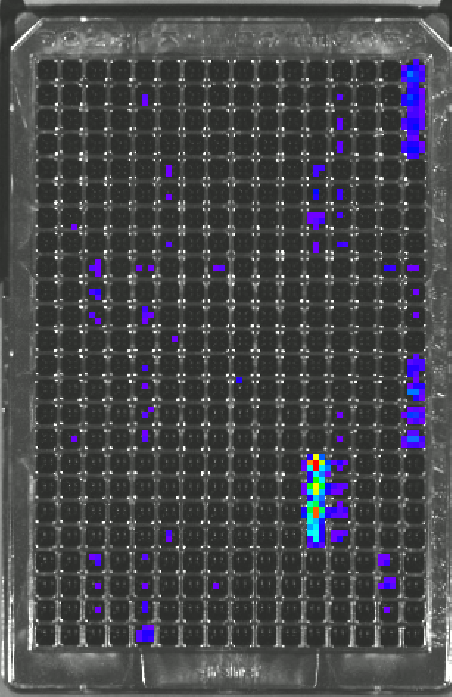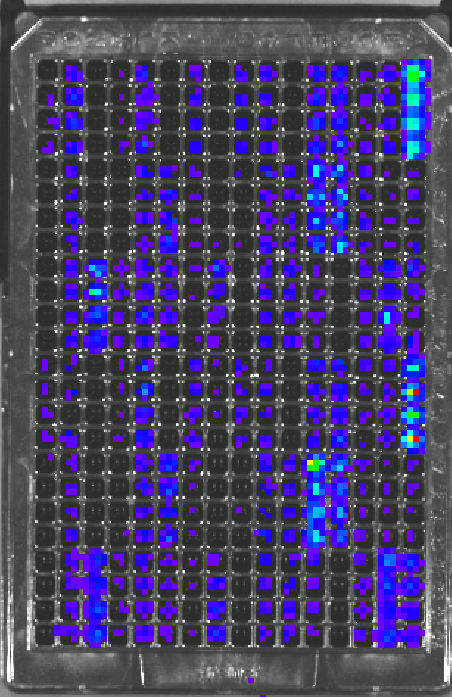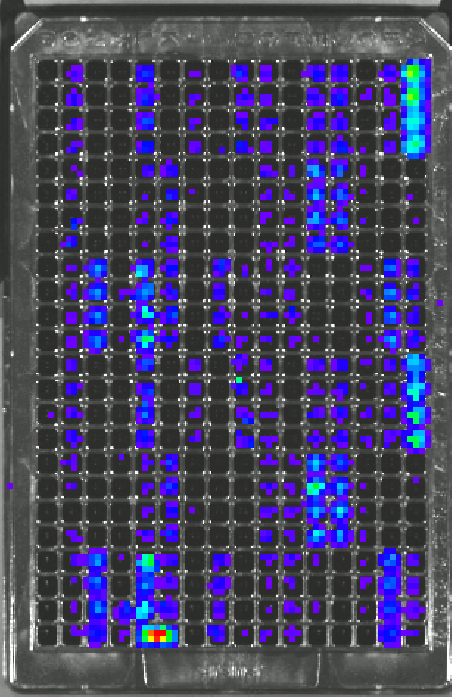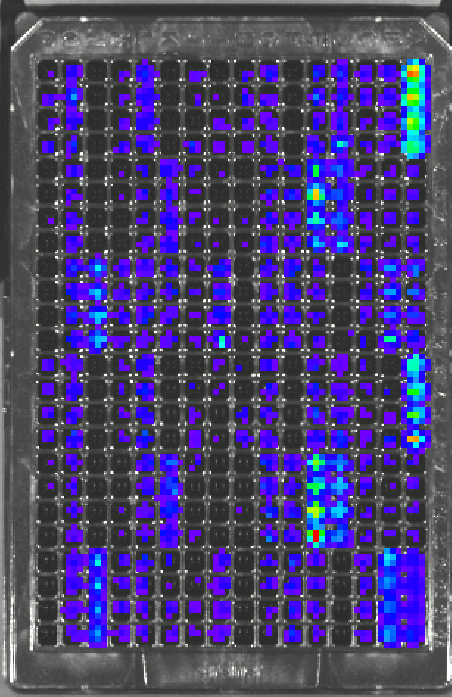
Transcription factors (TFs) are targeted for the quantification of activity as they are powerful regulators of cell differentiation, evidenced by the ability of a few TFs to reprogram fibroblasts to iPS cells. Furthermore, TFs are downstream targets of signaling pathways and large-scale TF quantification can reflect the activity throughout the intracellular signaling network. The large scale, dynamic, quantification of transcription factor (TF) activity is achieved in an array of cells, with each position of the array reporting on a distinct TF. Bioluminescence imaging (BLI) from a multi-well plate allows for rapid large-scale analysis. Additionally, BLI is non-invasive and allows for repeated measurements over time scales of minutes to weeks. Importantly, TF activity assays can be performed for cells cultured in our designer niches, which consist of tunable hydrogels that allow cells to grow in 3D and recreate the structures normally observed in vivo. An important aspect of this research is the development of computational models to analysis the dynamic TF data. This novel technology is central to the applications described below, including cancer biology, immunomodulation, and cell fate decisions.
Researchers: Joseph Decker, Jeffrey Ma